PPT Classification of Matter PowerPoint Presentation, free download
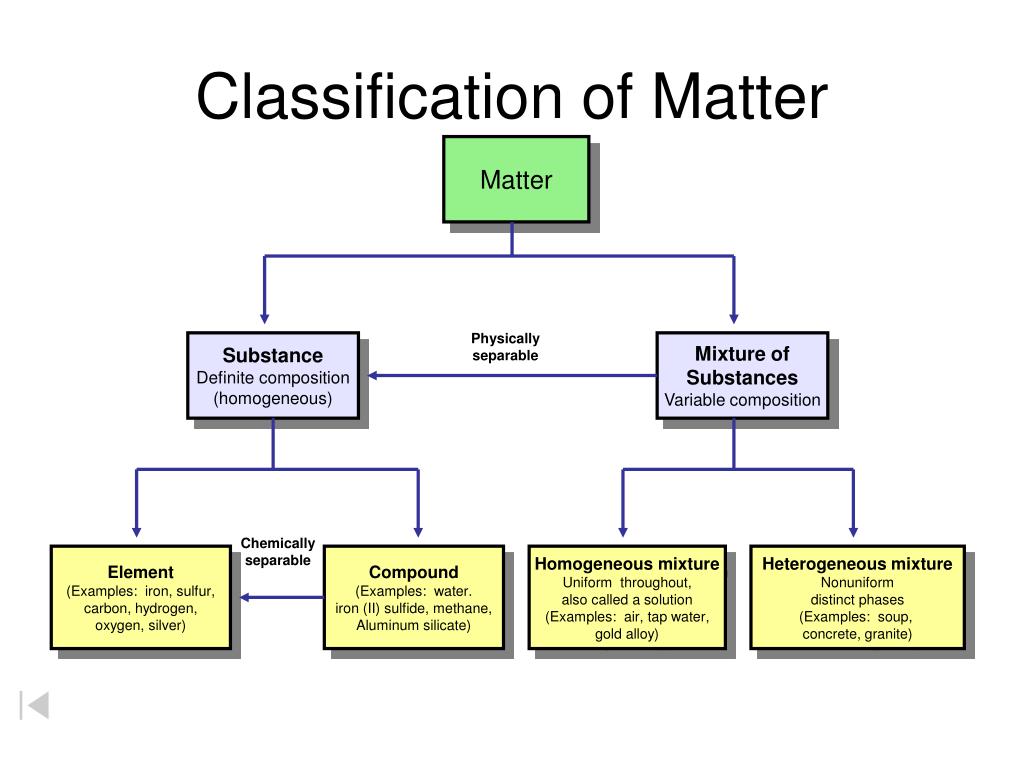
Classification of Matter. Unit 3 - Study of Matter. 2 of 32. What is Matter? Matter; The "stuff" that makes of the universe; Anything that has mass and takes up space; What are some examples of matter? 3 of 32. Properties of Matter.
PPT Classification of Matter PowerPoint Presentation, free download
A comprehensive 74-slide PPT product (with 4 pages of Summary Notes) that introduces all major topics in a typical middle school / early high school unit on classification of matter.Topics include: what is matter, atoms, pure substances, elements, compounds, mixtures, homogeneous, heterogenous, solutions, colloids, suspensions, separation techniques, physical and chemical properties of matter.

PPT Classification of Matter PowerPoint Presentation, free download
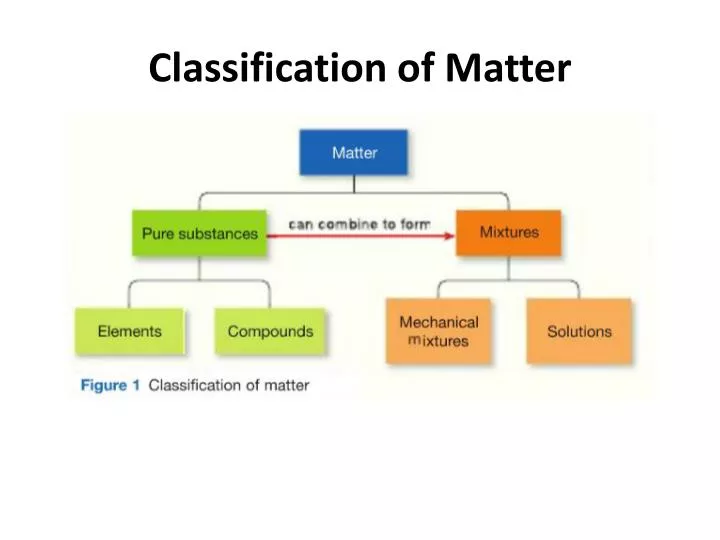
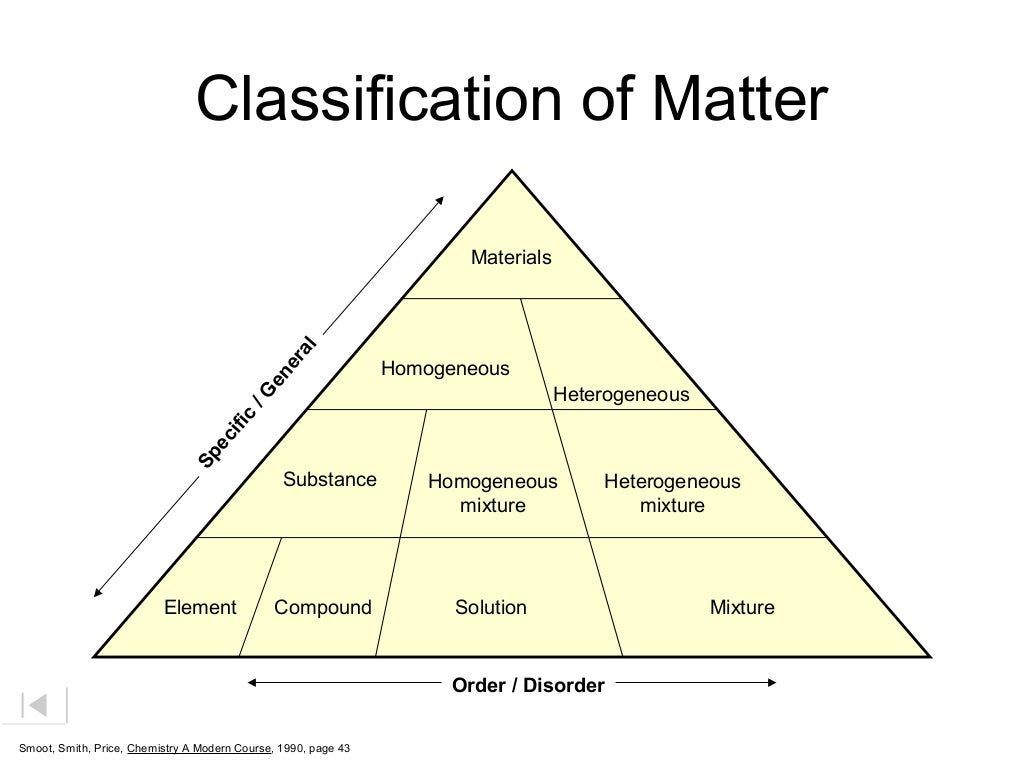
2. Elements only one kind of atom; atoms are bonded it the element is diatomic or polyatomic Compounds two or more kinds of atoms that are bonded substance with definite makeup and properties Mixtures two or more substances that are physically mixed two or more kinds of and Both elements and compounds have a definite makeup and definite.
PPT CLASSIFICATION OF MATTER PowerPoint Presentation, free download
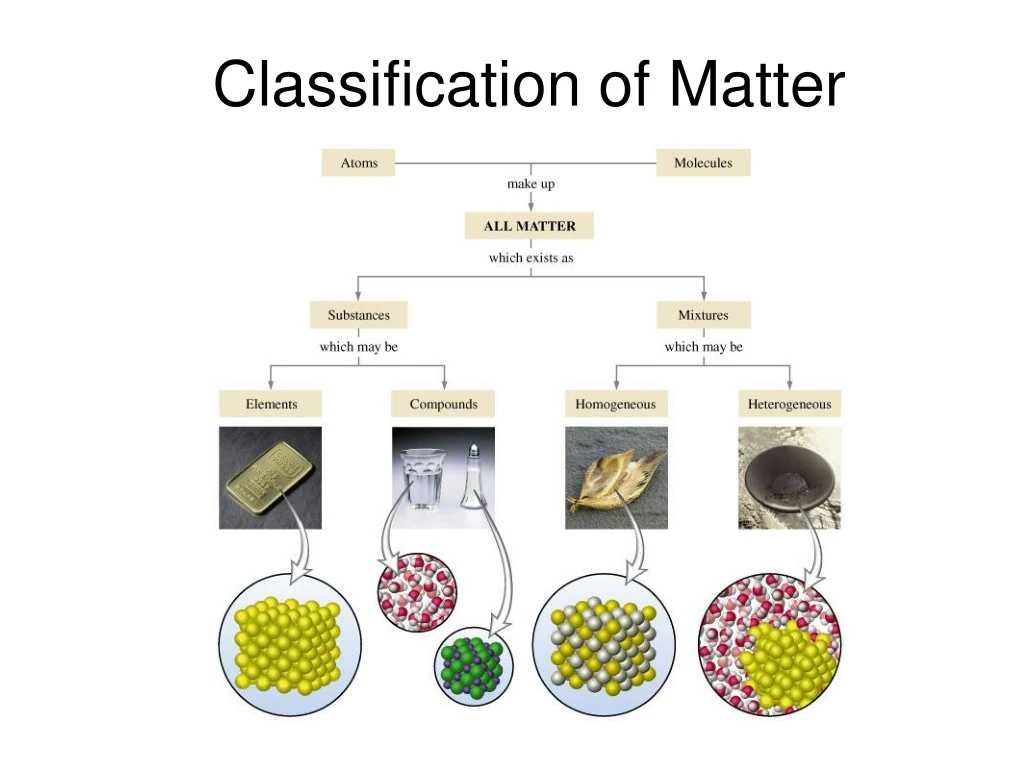
Classification of Pure Substances An element is a substance that cannot be chemically broken down into simpler substances. Basic building blocks of matter Composed of single type of atom, like helium A compound is a substance composed of two or more elements in fixed definite proportions.
PPT Classification of Matter PowerPoint Presentation, free download
Classification of Matter. Classification of Matter. Chapter 1 Chemistry 2A. Chemistry : the field of study concerned with the characteristics, composition, and transformations of matter Matter : anything that has mass and occupies space Living and non-living Macroscopic and microscopic. States of Matter. Solids. 305 views • 25 slides
Ch. 2 classification of matter ppt
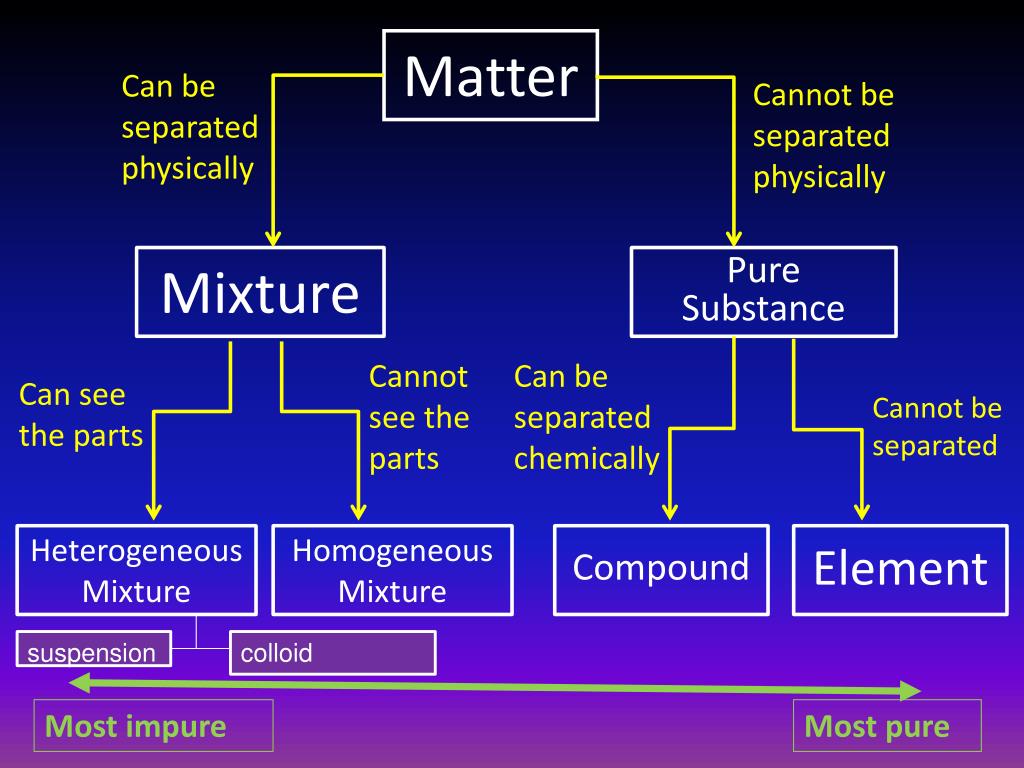
classification of matter: Oct 11, 2014 910 likes | 1.18k Views Chapter 5: Solutions. Classifying Solutions. 5.1- The Basics. classification of matter:. All Matter. Mixtures. Pure Substances. Heterogeneous (Mechanical Mixtures and Colloids). Homogeneous (Solutions). Elements. Compounds. is any that has. matter. solid, liquid or gas. mass.
PPT Classification of Matter PowerPoint Presentation, free download
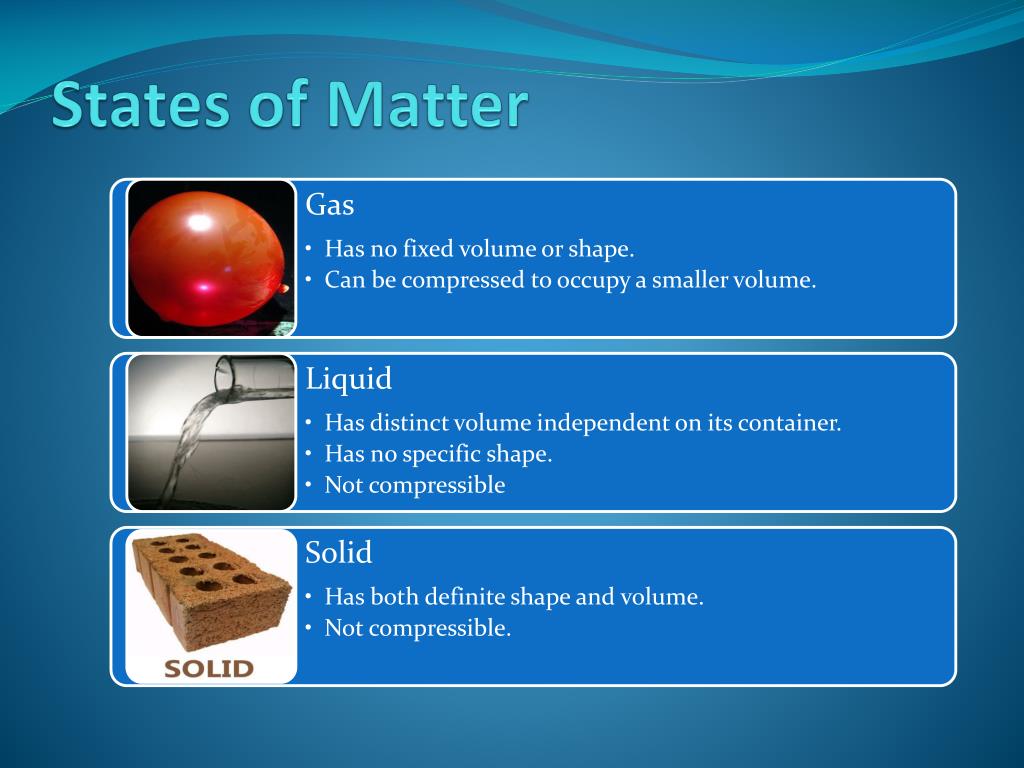
Physical properties are characteristics that describe matter. They include characteristics such as size, shape, color, and mass. Many of these properties can be quantitative in nature. For example, quantitative physical properties of water would be the boiling point (100 °C / 212 °F) and melting point (0°C / 32 °F).

5 1.1 Matter Powerpoint Part A Classification Of Matter
Classification of Matter Classification of Matter Physical Science Mixtures, elements, compounds Scientists like to classify things. One way that scientists classify matter is by its composition. Ultimately, all matter can be classified as mixtures, elements and compounds. Why isn't it a good idea to classify matter by its phases?
PPT Classification of Matter PowerPoint Presentation, free download
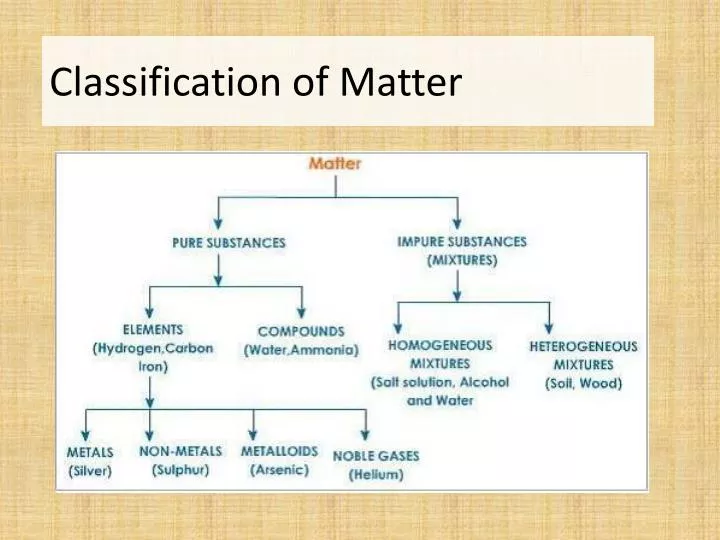
Kelompok 6 Tugas Akhir Pengantar Ekonomi Mikro Kelas H.pdf. Matter Mixture Pure Substance ElementCompoundHeterogeneous Mixture Homogeneous Mixture Can be separated physically Cannot be separated physically Can. 12. Pure Substances • Pure. Pure Substances • Elements. Classifications of Matter - Download as a PDF or view online for free.
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free
Dec 12, 2022 • 0 likes • 36 views Download Now Download to read offline Science Classification of matters L LinaAlKhalidi1 Recommended Balancing Equations Bruce Coulter 9.3K views • 23 slides Periodic table ppt cscope Jenny Dixon 30.3K views • 33 slides Acids & Bases OhMiss 40.5K views • 32 slides Elements, compound and mixture brintha smith

PPT Properties and classification of matter PowerPoint Presentation
2. Objectives Define matter. Explain the gas, liquid, and solid states of matter in terms of particles. Distinguish between the physical properties and chemical properties of matter. Classify Changes of matter as physical or chemical. Distinguish between a mixture and a pure substance. Identify the chemical symbols of elements, and name elements, given their symbols. Identify important.
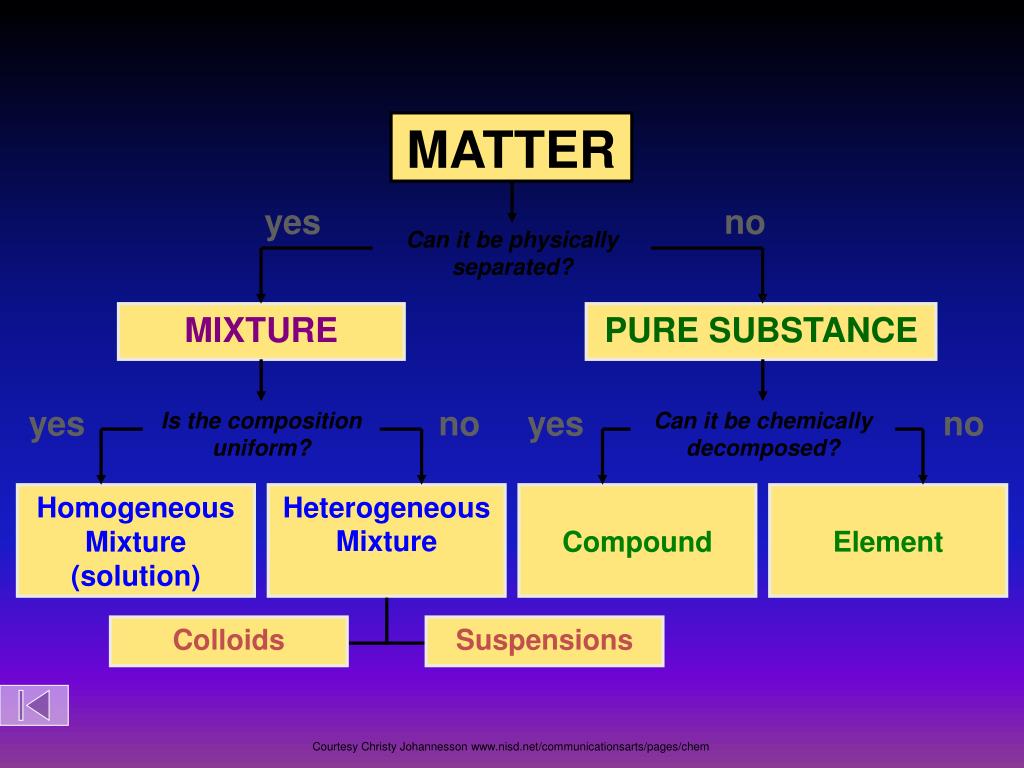
PPT Classification of Matter PowerPoint Presentation, free download
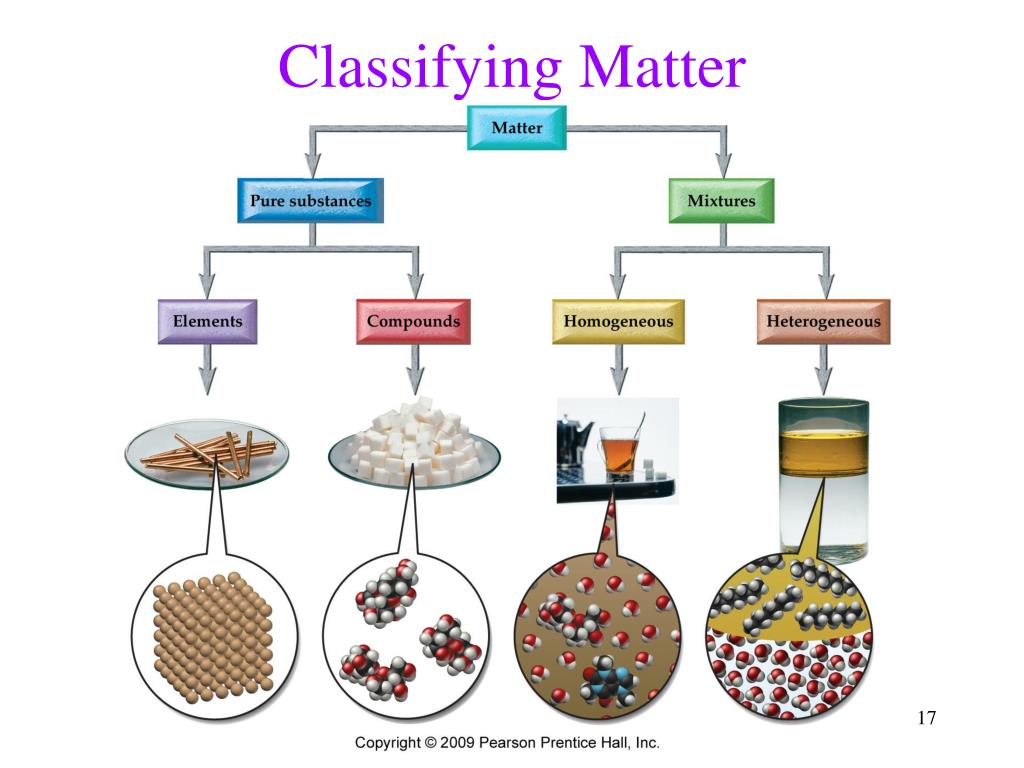
Properties of Matter. The classification of matter. Matter Mixtures Pure Substances. Pure Substances • A pure substance pure is made up of one type of particle (atoms or molecules) • Water • Chlorine • Oxygen. Mixtures • Contain 2+ different types of particles that do not chemically react.

PPT Classification of Matter PowerPoint Presentation, free download
Classification of Matter Matter Stuff of which all materials are made: anything that has mass and takes up space. Define Atoms- Extremely small building blocks of matter All matter is composed of atoms Atoms cannot be broken down into smaller pieces by chemical means The smallest distinct units in a sample of matter Elements are made up the.

PPT Classification of Matter PowerPoint Presentation, free download
3. Classifying Matter • *Matter is divided into two categories: mixtures and substances. • *Mixtures contain more than one kind of matter. - For example: Cola is a mixture of carbonated water, corn syrup, caramel color, phosphoric acid, natural flavors and caffeine.
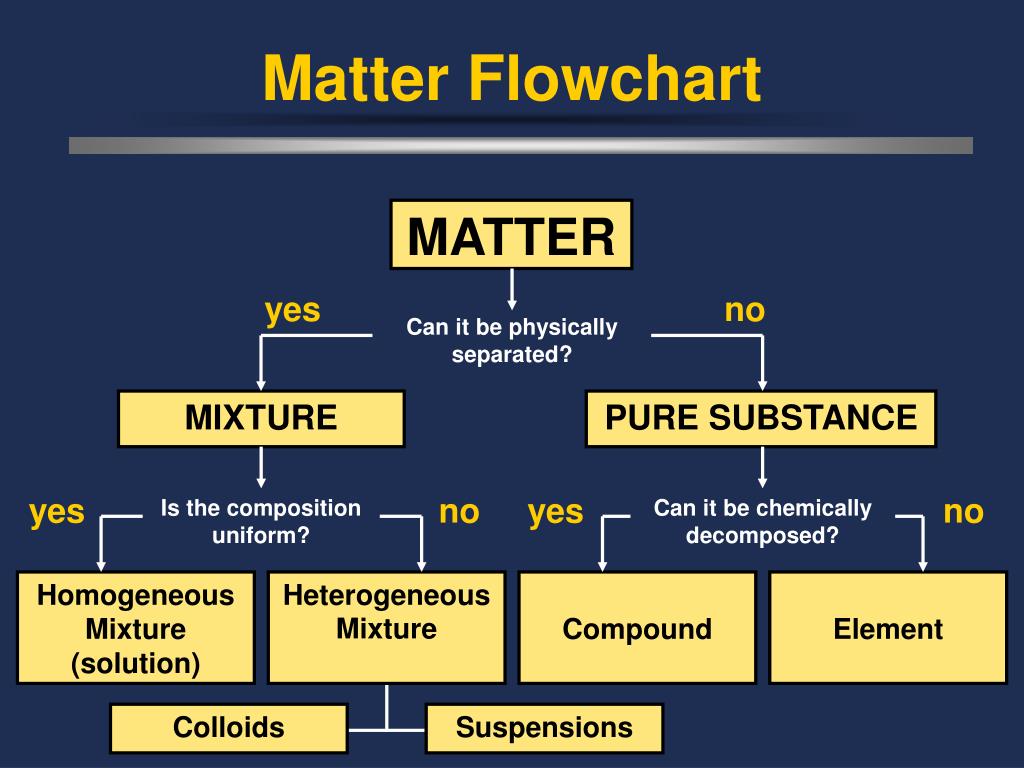
PPT Classification of Matter PowerPoint Presentation, free download
Classification of Matter Ordinary solid salt is a compound but not a molecule. It is built from interpenetrating lattices of sodium and chloride ions that extend indefinitely. This well-known molecule is a compound because it contains more than one element. A molecule but not a compound Ozone, O3, is not a compound because it contains only a.
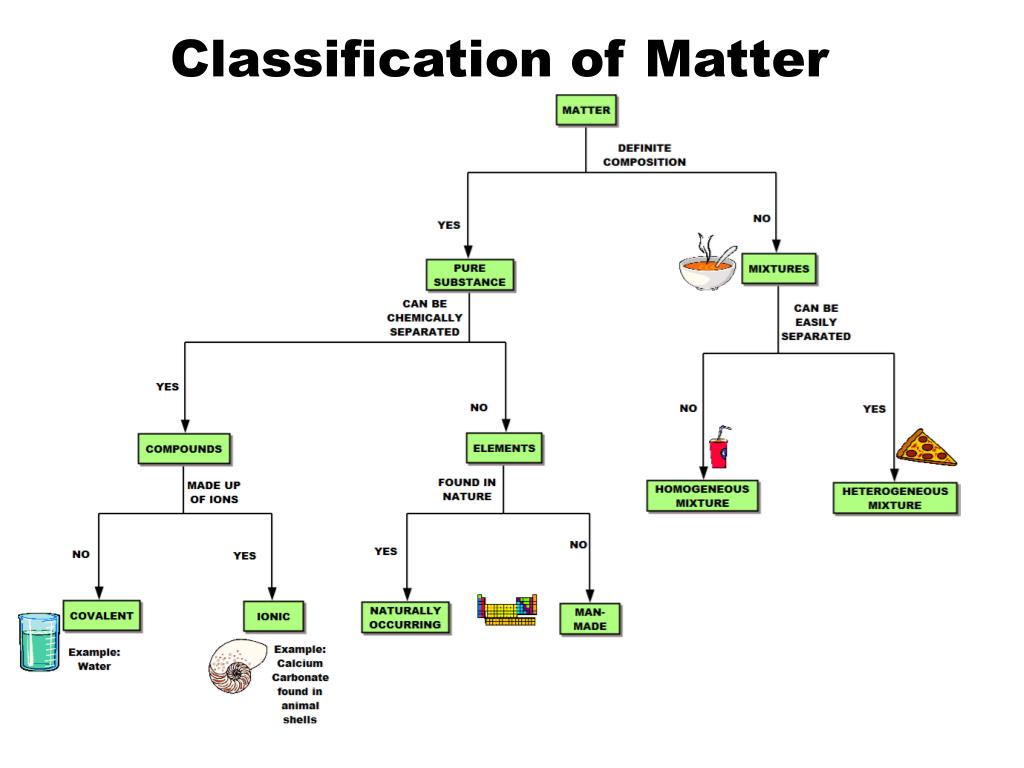
PPT Classification of Matter PowerPoint Presentation, free download
* Solution S1 Match: (1) solid, (2) liquid, or (3) gas. _2_ A. Has a definite volume, but shape of the container. _3_ B. Its particles are moving rapidly. _3_ C. Fills the volume of a container. _1_ D. Particles are in a fixed structure. _2_ E. Particles are close together, but mobile.